A team approach is essential when developing more effective treatments for ALS (amyotrophic lateral sclerosis), a progressive neurodegenerative disease affecting nerve cells in the brain and spinal cord. In her ongoing effort to determine the role of proteins in the disease and specifically why the neurons that affect movement are affected, Hande Ozdinler, PhD, associate professor of Neurology in the Feinberg School of Medicine, reached out to proteomics experts Neil Kelleher, PhD, the director of Northwestern Proteomics and the Chemistry of Life Processes Institute (CLP), and Steven Patrie, PhD, a research associate professor of Chemistry and director of Neuroproteomics within the Proteomics Center of Excellence, to find some answers.
“There’s something about collaborations,” says Ozdinler. “Experts in their own fields come together to answer even bigger questions.”
“We’re looking at the intact proteins from the top down,” Patrie says. This approach, which utilizes highly sophisticated mass spectrometry instruments, enables the analysis of intact forms of different proteins, or proteoforms, with complete molecular specificity. The more traditional ‘bottom-up’ method, on the other hand, breaks down proteins into pieces, making it very difficult to identify individual proteoforms that may be triggering the disease.
“We definitely bring something to the table at Northwestern Proteomics that is relatively unique to worldwide proteomics,” says Patrie.
To study proteins involved in ALS, the collaborators use immunoprecipitation with specific antibodies that target and attach to specific proteins, or as Patrie calls them, “molecular handles,” which enables their isolation and subsequent characterization via mass spectrometry. These antibodies target the proteins SOD-1 and TDP-43, which are known to play a role in ALS. The researchers have already had success targeting SOD-1 and are currently pursuing the same with TDP-43.\
The researchers are studying the TDP-43 protein in mouse brain tissue to identify unique variants of the protein. When TDP-43 protein mutations involved in ALS are put into mouse tissue, the tissue expresses the mutations as if they were in human tissue. These mutations occur when the proteins do not fold or group correctly. In these “humanized” mouse models, the team can study the progression of ALS in exactly the same way it would progress in a person. Ozdinler says they found that a mutation of TDP-43 is found in 90% of ALS patients.
In 2021, Kelleher and Ozdinler won a Cornew Innovation Award to pursue this research. The effort is part of a growing Neuroproteomics initiative headed by Patrie within the PCE. The award, says Ozdinler, supports collaborative research that aims to answer big scientific questions, such as what happens in the brain during ALS and what proteins are involved.
“We’re going to be quickly transitioning to the human tissue samples,” Patrie says. “That is definitely a driving force behind our research.
The collaborators’ work was also rewarded with an $800,000 US Department of Defense grant over two years to further extend their research. After researching human tissue samples, the team can start considering new approaches to treating ALS patients. By joining their backgrounds in neurology, chemistry and proteomics, they are optimistic about their chances for success.
“At the end of the day, I think our unique expertise will help us bring a mechanistic insight and also a path forward for revealing the underlying causes and building therapies,” Ozdinler said. “That’s why these collaborations are extremely important.”
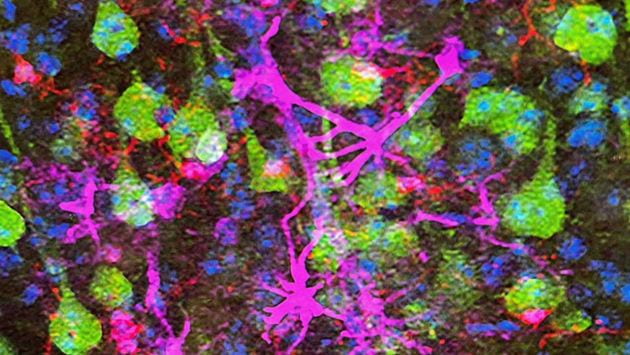
Main image: The neon green cells are the upper motor neurons, neurons that are impacted in ALS patients. Red- and magenta-colored cells are neuroimmune cells that contribute to neurodegeneration. (Source: Ozdinler Lab).
by Grace Finnell-Gudwien
Original article can be found here – https://www.clp.northwestern.edu/2022/10/27/cornew-innovation-award-collaborators-examine-the-role-of-proteins-in-als/

